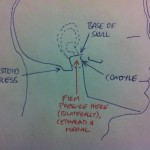
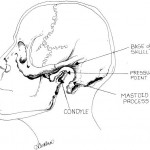
Larson’s Point for Laryngospasm
(Anesthesiology: November 1998 – Volume 89 – Issue 5 – p 1293–1294)
Planes of Anesthesia
Annals recs for credentialing, privileges, and practice (Ann Emerg Med 2011;58(4):365)
Who owns deep sedation editiorial (by Steve Green Ann Emerg Med 2011)
ASA Classification
- Class 1 Healthy patient, no medical problems
- Class 2 Mild systemic disease
- Class 3 Severe systemic disease, but not incapaitating
- Class 4 Severe systemic disease that is a constant threat to life
- Class 5 Moribund, not expected to live 24 hours irrespective of operation
- An e is added to the status number to designate an emergency operation. An organ donor is usually designated as Class 6
Levels of Sedation
Deep sedation by EPs is safe (W J EM 2011;12(4):399)
Fasting
review states only one case of aspiration in all of the literature (Emerg Med J 2010;27:254e261)
Peds
Fasting not necessary (Annals EM 42:5, November 2003)
Hemodynamic Characteristics
In healthy patients, midazolam had no effects
dex > propofol in BP reductions
Dex also reduced HR
(J Clin Anesth 2011;23:218)
Dexmedetomidine
Precedex
First case report (Eur J Emerg Med 2010;17(1):60)
Ketamine
New Annals Guidelines by Green & Krauss (Ann Emerg Med 2011; )
best article (Anaesthesia 2007;62(Sup 1):48-53
Ketamine Review from Pharm Journal
Green’s study of overdose show a remarkable degree of safety (ANNALS OF EMERGENCY MEDICINE 1999;34(4):492)
Krauss Free Emergency Lecture
neck/face rash common, not IgE mediated
1 pass drugs (propofol, etomidate, ketamine, sux, brevitol, thiopental, adenosine)
If pt has diplopia, ask them to close 1 eye
How Krauss gives ketamine to kids
0.5 mg/kg q 5 minutes x 3, maybe 4 doses for longer procedures
when the pt is >50 kg he doses lower. He gives 25 mg aliquots until the pt is where he wants.
IM Dosing: sub-disassoc 2mg/kg; disassoc 4-5 mg/kg
Is lamotrigine the antidote to the hallucinations (from ScanCrit pdf)
For perioperative pain (Anesth 2005;102:211) 0.5 mg/kg bolus and then same per hour
Ketamine Activates Breathing and Abolishes the Coupling between Loss of Consciousness and Upper
Airway Dilator Muscle Dysfunction in rats (ANESTHESIOLOGY 2012; 116:6–8.)
Preserves Laryngeal Reflexes (Anesthesiology 1973; 38:128 –33)
suggestion actually works to prevent ketamine bad trips (Anesthesia & Analgesia 2011;112(5):1082)
Case of laryngospasm (no actual spasm visualized) from prehospital ketamine (Burnett AM, PREHOSPITAL EMERGENCY CARE 2012;Early Online:1–3)
Review of pharm (Current Drug Targets, 2005, 6, 789-794)
alpha of 45 minutes
terminated by redistrib from cns and hepatic p450
increases CBF, so may increase CBV
A low dose infusion gives analgesia
Injections give analgesia and anxiolysis
High Doses give amnesia and disassociation
Cautions
· Central adrenergic release, premedication with depressants (benzos) or fentanyl will probably blunt this response.
· MAP increased ~25 mmHg
· Probably has neuroprotective effect by NDMA antagonism, so probably will be allowed to be used in stroke and head injury in the future.
· True laryngospasm is exceedingly rare, probably just tongue obstruction. Inevitably resolves with airway positioning.
· The intraocular pressure increase has only been reported in animals
· Avoid in hyperthyroid states due to catecholamine release
Premedication
· Glycopyrolate .01 mg/kg, not to exceed .2 mg or atropine .01 mg/kg not to exceed .5 mg (can go in same syringe as ketamine, though usually better to give 10-20 minutes beforehand)
· Benzos totally unnecessary in kids, probably not to be used for routine in adults as will prolong recovery times. Use when/if emergence reaction. Recovery period must be quiet, take off BP cuff, keep in calm environment.
Dosing
· IM 4 mg/kg (4-10) or for just analgesia 1 mg/kg
o Booster Doses 2-5 mg/kg q 10 minutes
o Use 100 mg/cc formulation
· Infusion is probably best route for adults
o Mix up bag 1 mg/cc
o .15 mg/kg/min until sedation then drop down to half that dose
· Injection 1 mg/kg (ETOH 3 mg/kg)
o .5-1 mg/kg booster doses q 10 minutes
o Give slowly to prevent apnea from blunted hypercapnia reflexes
Ketamine 1-2 mg/kg IV, give atropine beforehand (can go in same syringe), .5-1 mg/kg/hr infusion
Head Injury
Safety of sedation with ketamine and versed in severe head injury patients: comparison with sufentanil. No increases in ICP, comparable to the fentanyl derivative 25 patients (Crit Care Med. 2003 Mar;31(3):711-7)
and (Crit Care Med 2005;33(5):1109)
and (Ann Emerg Med 2015;65:43)
(Emergency Medicine Journal 2007;24:794-795) Cerebral blood flow (CBF) is critically dependant on cerebral perfusion pressure (CPP) and oxygenation in acute head injuries. Optimal CPP is achieved by maintaining a normal mean arterial pressure (MAP) and limiting iatrogenic increases in intracranial pressure (ICP).1 Brain tissue has high oxygen consumption and no reserves; hypoxia therefore has rapid and profound effects. Early tracheal intubation and ventilation can help prevent hypoxia and aspiration. Hypoxia and hypotension in traumatic brain injury are associated with a 75% mortality rate.2 End tidal carbon dioxide should be maintained around 5 kPa, as hypercapnia causes cerebral vasodilation and increased ICP.3 Ketamine, a potent analgesic, can be used for dissociative anaesthesia in higher doses (2 mg/kg), or sedation in lower doses. It has a rapid onset and relatively short duration of action (510 min). Unlike other commonly used induction agents, ketamine does not suppress respiratory activity or airway reflexes; it also has a positive effect on gut motility, and vomiting after administration is uncommon. These properties make it the ideal agent when profound analgesia and sedation are required without a definitive airway in place.3 Ketamine causes increased catecholamine release and decreased norepinephrine (noradrenaline) re-uptake which results in increased heart rate, arterial pressure, and MAP. This makes it a useful analgesic for trauma patients who may already be haemodynamically compromised. A single episode of hypotension is associated with a worse outcome.2 Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist. Head injury increases concentrations of glutamate, which induces neuronal apoptosis. Ketamine blocks the actions of glutamate on the NMDA receptor, which may protect against cellular neurotoxicity, but this has yet to be demonstrated in human studies.1 3 4 Despite these benefits, the use of ketamine in patients with head injuries remains controversial. Early studies suggested that the use of ketamine may have resulted in a transient increase in ICP in a small number of patients.3 CPP was compromised only in the patients with pre-existing intracranial hypertension and obstruction to the flow of cerebral spinal fluid. This has, however, led to the persistent belief that ketamine is contraindicated in patients with traumatic head injuries. Studies done subsequently have shown, however, that the effects of ketamine on cerebral haemodynamics and ICP are in fact variable and depend on both the presence of additional anaesthetic agents and PaCO2 values.5 This patient was sedated with midazolam 10 mg iv, which prevents the emergence phenomenon, and ventilation was controlled artificially en route to hospital. When ketamine is used in the presence of controlled ventilation, in conjunction with anaesthetics which reduce cerebral metabolism such as {gamma}-aminobutyric acid (GABA) receptor agonists, ICP is not increased.1 4 1. Albanèse J, Arnaud S, Rey M, et al. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology 1997; 87: 132834.[CrossRef][Medline] 2. Anon. Prehospital Trauma Life Support Committee of the National Association of Emergency Medical Technicians in Cooperation with The Committee on Trauma of The American College of Surgeons. PHTLS , 6th ed, 2007: 194221. 3. Sehdev RS, Symmons DAD, Kindl K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas 2006; 18: 3744.[CrossRef][Medline] 4. Himmelseher S, Durieux ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg 2005; 101: 52434.[Abstract/Free Full Text] 5. Mayberg TS, Lam AM, Matta BF et al. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anaesthesia in patients undergoing craniotomy. Anesth Analg 1995; 81: 849.[Abstract]
J Neurosurg Pediatr. 2009 Jul;4(1):40-6.LinksEffectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. Bar-Joseph G, Guilburd Y, Tamir A, Guilburd JN.Paediatric Critical Care and.Object Deepening sedation is often needed in patients with intracranial hypertension. All widely used sedative and anesthetic agents (opioids, benzodiazepines, propofol, and barbiturates) decrease blood pressure and may therefore decrease cerebral perfusion pressure (CPP). Ketamine is a potent, safe, rapid-onset anesthetic agent that does not decrease blood pressure. However, ketamine’s use in patients with traumatic brain injury and intracranial hypertension is precluded because it is widely stated that it increases intracranial pressure (ICP). Based on anecdotal clinical experience, the authors hypothesized that ketamine does not increase-but may rather decrease-ICP. Methods The authors conducted a prospective, controlled, clinical trial of data obtained in a pediatric intensive care unit of a regional trauma center. All patients were sedated and mechanically ventilated prior to inclusion in the study. Children with sustained, elevated ICP (> 18 mm Hg) resistant to first-tier therapies received a single ketamine dose (1-1.5 mg/kg) either to prevent further ICP increase during a potentially distressing intervention (Group 1) or as an additional measure to lower ICP (Group 2). Hemodynamic, ICP, and CPP values were recorded before ketamine administration, and repeated-measures analysis of variance was used to compare these values with those recorded every minute for 10 minutes following ketamine administration. Results The results of 82 ketamine administrations in 30 patients were analyzed. Overall, following ketamine administration, ICP decreased by 30% (from 25.8 +/- 8.4 to 18.0 +/- 8.5 mm Hg) (p < 0.001) and CPP increased from 54.4 +/- 11.7 to 58.3 +/- 13.4 mm Hg (p < 0.005). In Group 1, ICP decreased significantly following ketamine administration and increased by > 2 mm Hg during the distressing intervention in only 1 of 17 events. In Group 2, when ketamine was administered to lower persistent intracranial hypertension, ICP decreased by 33% (from 26.0 +/- 9.1 to 17.5 +/- 9.1 mm Hg) (p < 0.0001) following ketamine administration. Conclusions In ventilation-treated patients with intracranial hypertension, ketamine effectively decreased ICP and prevented untoward ICP elevations during potentially distressing interventions, without lowering blood pressure and CPP. These results refute the notion that ketamine increases ICP. Ketamine is a safe and effective drug for patients with traumatic brain injury and intracranial hypertension, and it can possibly be used safely in trauma emergency situations.
Clinical guidelines in Peds (Ann Emerg Med. 2004;44:460-471)
Probably no difference with or without antisalagogue (Acad Emerg Med. 2003;10:482-483.)
Case series of use in mentally disabled adults (Acad Emerg Med 1999 6(1):86)
Purpose To define the guidelines for patient selection, administration, monitoring, and recovery for ED dissociative sedation. Definition of Dissociative Sedation d A trancelike cataleptic state induced by the dissociative agent ketamine, characterized by profound analgesia and amnesia, with retention of protective airway reflexes, spontaneous respirations, and cardiopulmonary stability. Characteristics of the Ketamine Dissociative State d Dissociation: After administration of ketamine, the patient passes into a fugue state or trance. The eyes may remain open, but the patient does not respond. d Catalepsy: Normal or slightly enhanced muscle tone is maintained. On occasion, the patient may move or be moved into a position that is selfmaintaining. Occasional muscular clonus may be noted. d Analgesia: Analgesia is typically substantial or complete. d Amnesia: Total amnesia is typical. d Maintenance of airway reflexes: Upper airway reflexes remain intact and may be slightly exaggerated. Intubation is unnecessary, but occasional repositioning of the head may be necessary for optimal airway patency. Suctioning of hypersalivation may occasionally be necessary. d Cardiovascular stability: Blood pressure and pulse rate are not decreased and typically are mildly increased. d Nystagmus: Nystagmus is typical. Indications d Short, painful procedures, especially those requiring immobilization (eg, facial laceration, burn debridement, fracture reduction, abscess incision and drainage, central line placement, tube thoracostomy). d Examinations judged likely to produce excessive emotional disturbance (eg, pediatric sexual assault examination). Contraindications: Absolute (Risks Essentially Always Outweigh Benefits) d Age younger than 3 months (higher risk of airway complications) d Known or suspected psychosis, even if currently stable or controlled with medications (can exacerbate condition) Contraindications: Relative (Risks May Outweigh Benefits) d Aged 3 to 12 months (higher risk of airway complications) d Procedures involving stimulation of the posterior pharynx (higher risk of laryngospasm) d History of airway instability, tracheal surgery, or tracheal stenosis (presumed higher risk of airway complications) d Active pulmonary infection or disease, including upper respiratory infection or asthma (higher risk of laryngospasm) d Known or suspected cardiovascular disease, including angina, heart failure, or hypertension (exacerbation due to sympathomimetic properties of ketamine). Avoid ketamine in patients who are already hypertensive and in older adults with risk factors for coronary artery disease. d Head injury associated with loss of consciousness, altered mental status, or emesis (elevated intracranial pressure with ketamine) d Central nervous system masses, abnormalities, or hydrocephalus (elevated intracranial pressure with ketamine) d Glaucoma or acute globe injury (elevated intraocular pressure with ketamine) d Porphyria, thyroid disorder, or thyroid medication (enhanced sympathomimetic effect) Personnel d Dissociative sedation is a 2-person procedure, 1 (eg, nurse) to monitor the patient and 1 (eg, physician) to perform the procedure. Both must be knowledgeable about the unique characteristics of ketamine. d Avoid dissociative sedation when personnel are not experienced with ketamine or may not have time to perform such sedation properly. Presedation d Perform a standard presedation assessment d Educate accompanying family about the unique characteristics of the dissociative state, especially if they will be present during the procedure or recovery.
Atropine and Glycopyrrolate glycopyrrolate 0.2 mg both pregnancy class B
Emergence Reaction
Hatzskorzian R, Li Pi Shan W, Côté AV, Schricker T, Backman SB. The management of severe emergence agitation using droperidol. Anaesthesia 2006; 61: 11125. 2 Malviya S, Voepel-Lewis T, Ramamurthi R, Burke C, Tait AR. Clonidine for the prevention of emergence agitation in young children: efficacy and recovery profile. Pediatric Anesthesia 2006; 16: 5549.
Study results were published in the August issue of the Archives of General Psychiatry. “The public health implications of being able to treat major depression this quickly would be enormous,” said NIH Director Elias A. Zerhouni, M.D. “These new findings demonstrate the importance of developing new classes of antidepressants that are not simply variations of existing medications.” For this study 18 treatment-resistant, depressed patients were randomly assigned to receive either a single intravenous dose of ketamine or a placebo (inactive compound). Depression improved within one day in 71 percent of all those who received ketamine, and 29 percent of these patients became nearly symptom-free within one day. Thirty-five percent of patients who received ketamine still showed benefits seven days later. Participants receiving a placebo infusion showed no improvement. One week later, participants were given the opposite treatment, unless the beneficial effects of the first treatment were still evident. This “crossover” study design strengthens the validity of the results.
56 C.R. Chudnofsky, J.E. Weber and P.J. Stoyanoff et al., A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients, Acad Emerg Med 7 (2000), pp. 228235. Abstract + References in Scopus | Cited By in Scopus
57 S.M. Green and J. Li, Ketamine in adults: what emergency physicians need to know about patient selection and emergence reactions [editorial], Acad Emerg Med 7 (2000), pp. 278281. Abstract + References in Scopus | Cited By in Scopus
RCT of midazolam, NNT of 6 to avoid emergence. They used 0.03 mg/kg of the midaz (Ann Emerg Med 2011;57:109)
Ketamine reduces morphine consumption (Am J Emerg Med 2007;25:385)
Scott Weingart <emcrit@gmail.com> Thu, Jul 17, 2008 at 4:23 AM Reply-To: emcrit@gmail.com To: Scott Weingart <scott.weingart@mssm.edu> Induction phase shoot for 30 minutes 35 no bleeding risk 33.5 coagulopathy PCIM 16 44.1 hz post resus cardioprotective procoagulant ischemic/reperfusion Ketamine.
Historically, ketamine has played a central role in anesthesia for the trauma patient as a result of the profound analgesia and hemodynamic stability it provides. Increasingly, ketamine has been used for postoperative analgesia and acute pain management in the trauma patient; the Army has for years been interested in developing a nasal formulation of ketamine specifically for acute pain. The Army has accrued significant experience in low-dose ketamine in both acute and chronic pain. Even at subanalgesic doses, it appears to improve fentanyl’s efficacy in certain pain domains (54, 131).
The most publicized adverse effect of ketamine has been its psychotomimetic effects. Combining ketamine with other agents, such as benzodiazepines or propofol, has been found to attenuate and often abolish the psychotomimetic side effects (132, 133). A series by Friedberg evaluated the use of ketamine, combined with propofol, in 1264 patients premedicated with benzodiazepines and reported no emergence dreams or hallucinations (134). Several studies demonstrate the occurrence of psychotomimetic effects with ketamine is directly related to plasma concentrations of the drug (135, 136). Analgesic plasma concentrations are lower than the plasma concentrations seen with psychotomimetic effects (54, 131).
Long-lasting psychiatric effects have also been a concern surrounding ketamine use. Previous Air Force policy stated that ketamine administration was a nonwaiverable event for flying status. Hersack, in 1994, reviewed the literature for the incidence of long-term side effects and found ketamine had no long-term psychologic effects (137). The only serious sequelae were four cases of psychologic effects lasting up to 3 wks with subsequent resolution. Henceforth, the Air Force changed its policy on ketamine, shortening restricted flying status for the first 3 wks after ketamine administration (137).
A second factor impeding the use of ketamine is purported increases in intracranial pressure (ICP). However, studies supporting this hypothesis were performed in spontaneously ventilating subjects in which the Paco2 was not controlled (138, 139). Research performed in subjects administered ketamine under controlled ventilation demonstrated no evidence of increases in cerebral blood flow or intracranial pressure when CO2 was held constant (140, 141).
Another proposed mechanism of increased ICP secondary to ketamine was a postulated direct dilatory effect on cerebral vasculature (142). Schwedler et al. injected ketamine directly into the cerebral circulation and failed to produce significant change in cerebral blood flow (141). Nonetheless, the current teaching is that ketamine should be avoided in patients with intracranial pathology because it may elevate ICP. When multiple studies enrolling patients with increased ICP were performed, ketamine demonstrated an attenuation of further increases in ICP when administered in combination with a benzodiazepine (143, 144). Patients with traumatic brain injury were also studied, and ketamine demonstrated no adverse effects on cerebral hemodynamics; in fact, decreased ICP was observed in the ketamine group (145).
Another criticism of ketamine is its alleged depressant actions on myocardial tissue, thereby decreasing its use in the patient with a catecholamine-depleted state. This misconception is derived from a study of in vitro canine atrial tissue, which demonstrated negative inotropic actions at high plasma concentrations of ketamine (10300 µg/mL) (146). However, clinically used ketamine levels, in the same study (less than 3 µg/mL), displayed positive inotropic effects. These early studies also failed to adequately evaluate ketamine’s cardiovascular action in vivo on human subjects at clinically used levels. Many intravenous anesthetics evaluated in human myocardial tissue, including etomidate, propofol, thiopental, and midazolam, found ketamine to be the least depressant on myocardial tissue (147). Ketamine was found to have less negative inotropic effect on the myocardium than etomidate, a widely accepted induction agent for patients with cardiovascular failure. Ketamine is thought to act as a cardiac stimulant through sympathetic-mediated mechanisms.
Cardiac stimulation, however, is not always desired, especially in patients with increased myocardial oxygen demand and limited supply such as those with coronary artery disease. Premedication, especially with benzodiazepines, has been found to reliably attenuate the stimulating cardiovascular effects of ketamine (148). Interestingly, a recent study comparing ketamine with an inhalational/opioid technique in coronary artery surgery found ketamine use decreased the need for inotropes after surgery and reduced the incidence of myocardial infarctions (149).
Ketamine’s use throughout the “inflammatory period” of injury may result in decreased central hypersensitivity resulting from the continual C fiber windup phenomenon in the polytrauma patient. Ketamine binds noncompetitively to the phencyclidine site of the NMDA receptor as well as sigma opioid receptor resulting in intense analgesia; other benefits include prevention of OIH, decreased opioid tolerance, decreased opioid requirements, increased sense of well-being and patient satisfaction, decreased risk of respiratory depression, and decreased chronic pain. Although anesthetic doses may be associated with secretions as well as agitation and hallucinations, subanesthetic doses are tolerated extremely well with the addition of a benzodiazepine if necessary. The combination of ketamine and morphine in low PCA doses (1 mg morphine and 1 mg ketamine) has been shown to be beneficial with few side effects (150). Ketamine infusions below 2.5 µg/kg/min have also shown similar benefits in reducing opioid consumption and having few side effects (131, 151).
54. Tucker A, Kim YI, Nadeson R, et al: Investigation of the potentiation of the analgesic effects of fentanyl by ketamine in humans: A double-blinded, randomised, placebo controlled, crossover study of experimental pain. BMC Anesthesiology 2005; 5:2 [Context Link]
55. Koppert W, Sittl R, Scheuber K, et al: Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology 2003; 99:152159 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
56. Reuben SS, Buvanendran A: Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am 2007; 89:13431358 [Context Link]
57. Reuben SS, Ekman EF: The effect of initiating a preventive multimodal analgesic regimen on long-term patient outcomes for outpatient anterior cruciate ligament reconstruction surgery. Anesth Analg 2007; 105:228232 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
58. Angst MS, Clark JD: Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006; 104:570587 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
59. Celerier E, Rivat C, Jun Y, et al: Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology 2000; 92:465472 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
60. Hudcova J, McNicol E, Quah C, et al: Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2006; 4:CD003348 [Context Link]
61. American Society of Anesthesiologists Task Force on Acute Pain: Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2004; 100:15731581 Mount Sinai Serials [Context Link]
62. Werner MU, Soholm L, Rotboll-Nielsen P, et al: Does an acute pain service improve postoperative outcome? Anesth Analg 2002; 95:13611372 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
63. Ong BY, Arneja A, Ong EW: Effects of anesthesia on pain after lower-limb amputation. J Clin Anesth 2006; 18:600604 [Context Link]
64. Richman JM, Liu SS, Courpas G, et al: Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006; 102:248257 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
65. Schulz-Stubner S, Boezaart A, Hata JS: Regional analgesia in the critically ill. Crit Care Med 2005; 33:14001407 Ovid Full Text Mount Sinai Serials [Context Link]
66. Pogatzki-Zahn EM, Zahn PK: From preemptive to preventive analgesia. Curr Opin Anaesthesiol 2006; 19:551555 Ovid Full Text Mount Sinai Serials [Context Link]
67. Moiniche S, Kehlet H, Dahl JB: A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology 2002; 96:725741 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
68. Grass J: Surgical outcome: regional anesthesia and analgesia versus general anesthesia. Anesthesia Review 1993; 20:117125 [Context Link]
69. Guinard JP, Mavrocordatos P, Chiolero R, et al: A randomized comparison of intravenous versus lumbar and thoracic epidural fentanyl for analgesia after thoracotomy. Anesthesiology 1992; 77:11081115 Mount Sinai Serials Bibliographic Links [Context Link]
70. Boylan JF, Katz J, Kavanagh BP, et al: Epidural bupivacaine-morphine analgesia versus patient-controlled analgesia following abdominal aortic surgery: Analgesic, respiratory, and myocardial effects. Anesthesiology 1998; 89:585593 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
71. Rawal N, Sjostrand U, Christoffersson E, et al: Comparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese: Influence on postoperative ambulation and pulmonary function. Anesth Analg 1984; 63:583592 Ovid Full Text Mount Sinai Serials [Context Link]
72. Salomaki TE, Laitinen JO, Nuutinen LS: A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after thoracotomy. Anesthesiology 1991; 75:790795 [Context Link]
73. Liu SS, Wu CL: Effect of postoperative analgesia on major postoperative complications: A systematic update of the evidence. Anesth Analg 2007; 104:689702 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
74. Ford RP, Gerancher JC, Rich R, et al: An evaluation of immediate recovery after regional and general anesthesia: A two-year review of 801 ambulatory patients undergoing hand surgery. Reg Anesth Pain Med 2001; 26(Suppl):42 [Context Link]
75. Yeager MP, Glass DD, Neff RK, et al: Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology 1987; 66:729736 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
76. Blomberg S, Emanuelsson H, Kvist H, et al: Effects of thoracic epidural anesthesia on coronary arteries and arterioles in patients with coronary artery disease. Anesthesiology 1990; 73:840847 [Context Link]
77. Blomberg S, Emanuelsson H, Ricksten SE: Thoracic epidural anesthesia and central hemodynamics in patients with unstable angina pectoris. Anesth Analg 1989; 69:558562 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
78. Gramling-Babb P, Miller MJ, Reeves ST, et al: Treatment of medically and surgically refractory angina pectoris with high thoracic epidural analgesia: Initial clinical experience. Am Heart J 1997; 133:648655 Mount Sinai Serials Buy Now Bibliographic Links [Context Link]
79. Scott NB, Turfrey DJ, Ray DA, et al: A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg 2001; 93:528535 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
80. Liu SS, Block BM, Wu CL: Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: A meta-analysis. Anesthesiology 2004; 101:153161 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
81. Gold MS, DeCrosta D, Rizzuto C, et al: The effect of lumbar epidural and general anesthesia on plasma catecholamines and hemodynamics during abdominal aortic aneurysm repair. Anesth Analg 1994; 78:225230 Mount Sinai Serials Bibliographic Links [Context Link]
82. Benzon HT, Wong CA, Wong HY, et al: The effect of low-dose bupivacaine on postoperative epidural fentanyl analgesia and thrombelastography. Anesth Analg 1994; 79:911917 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
83. Liu SS, Richman JM, Thirlby RC, et al: Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: A quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006; 203:914932 Mount Sinai Serials [Context Link]
84. Capdevila X, Barthelet Y, Biboulet P, et al: Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999; 91:815 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
85. Singelyn FJ, Gouverneur JM: Postoperative analgesia after total hip arthroplasty: i.v. PCA with morphine, patient-controlled epidural analgesia, or continuous ‘3-in-1’ block?: A prospective evaluation by our acute pain service in more than 1,300 patients. J Clin Anesth 1999; 11:550554 Mount Sinai Serials [Context Link]
86. Borgeat A, Perschak H, Bird P, et al: Patient-controlled interscalene analgesia with ropivacaine 0.2% versus patient-controlled intravenous analgesia after major shoulder surgery: Effects on diaphragmatic and respiratory function. Anesthesiology 2000; 92:102108 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
87. Wu CL, Naqibuddin M, Fleisher LA: Measurement of patient satisfaction as an outcome of regional anesthesia and analgesia: a systematic review. Reg Anesth Pain Med 2001; 26:196208 Mount Sinai Serials Bibliographic Links [Context Link]
88. Auroy Y, Benhamou D, Bargues L, et al: Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service [erratum appears in Anesthesiology 2003;98:595 Note: Mercier Frederic {corrected to Mercier Frederic J}]. Anesthesiology 2002; 97:12741280 [Context Link]
89. Greensmith JE, Murray WB: Complications of regional anesthesia. Curr Opin Anaesthesiol 2006; 19:531537 [Context Link]
90. Brown DL, Ransom DM, Hall JA, et al: Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg 1995; 81:321328 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
91. Foxall G, McCahon R, Lamb J, et al: Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia 2007; 62:516518 [Context Link]
92. Litz RJ, Popp M, Stehr SN, et al: Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia 2006; 61:800801 Mount Sinai Serials Buy Now [Context Link]
93. Faccenda KA, Finucane BT: Complications of regional anaesthesia. Incidence and prevention. Drug Saf 2001; 24:413442 Mount Sinai Serials Buy Now Bibliographic Links [Context Link]
94. Stan TC, Krantz MA, Solomon DL, et al: The incidence of neurovascular complications following axillary brachial plexus block using a transarterial approach. A prospective study of 1,000 consecutive patients. Reg Anesth 1995; 20:486492 Mount Sinai Serials Bibliographic Links [Context Link]
95. Borgeat A, Ekatodramis G, Kalberer F, et al: Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001; 95:875880 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
96. Bergman BD, Hebl JR, Kent J, et al: Neurologic complications of 405 consecutive continuous axillary catheters. Anesth Analg 2003; 96:247252 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
97. Franco CD, Vieira ZE: 1,001 subclavian perivascular brachial plexus blocks: Success with a nerve stimulator. Reg Anesth Pain Med 2000; 25:4146 Mount Sinai Serials Bibliographic Links [Context Link]
98. Brown DL, Cahill DR, Bridenbaugh LD: Supraclavicular nerve block: Anatomic analysis of a method to prevent pneumothorax. Anesth Analg 1993; 76:530534 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
99. Lonnqvist PA, MacKenzie J, Soni AK, et al: Paravertebral blockade. Failure rate and complications. Anaesthesia 1995; 50:813815 Mount Sinai Serials [Context Link]
100. Horlocker T (ed): Regional Anesthesia and Anticoagulation: Are the Benefits Worth the Risks? Regional Anesthesia for Cardiothoracic Surgery. Baltimore, Lippincott Williams and Wilkins, 2002 [Context Link]
101. Klein SM, D’Ercole F, Greengrass RA, et al: Enoxaparin associated with psoas hematoma and lumbar plexopathy after lumbar plexus block. Anesthesiology 1997; 87:15761579 [Context Link]
102. Feldman JM, Chapin-Robertson K, Turner J: Do agents used for epidural analgesia have antimicrobial properties? Reg Anesth 1994; 19:4347 Mount Sinai Serials Bibliographic Links [Context Link]
103. Aydin ON, Eyigor M, Aydin N: Antimicrobial activity of ropivacaine and other local anaesthetics. Eur J Anaesthesiol 2001; 18:687694 Mount Sinai Serials Bibliographic Links [Context Link]
104. Marret E, Remy C, Bonnet F: Postoperative Pain Forum G. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg 2007; 94:665673 [Context Link]
105. Ganesh A, Cucchiaro G: Multiple simultaneous perineural infusions for postoperative analgesia in adolescents in an outpatient setting. Br J Anaesth 2007; 98:687689 [Context Link]
106. McQuay H: Opioids in pain management. Lancet 1999; 353:22292232 Mount Sinai Serials Bibliographic Links [Context Link]
107. Faura CC, Collins SL, Moore RA, et al: Systematic review of factors affecting the ratios of morphine and its major metabolites. Pain 1998; 74:4353 Mount Sinai Serials Bibliographic Links [Context Link]
108. Miniño AM, Anderson RN, Fingerhut LA, et al: Increases in Methadone-Related Deaths: 19992004. Statistics NCfH (Ed). Volume 54. Atlanta, Centers for Disease Control and Prevention, 2006 [Context Link]
109. Center for Substance Abuse Treatment M-AMRoaN, Assessment M-, 2003. SAMHSA Publication No. 04-3904. Rockville, MD, Center for Substance Abuse Treatment SAaMHSA, 2004 [Context Link]
110. Ehret GB, Voide C, Gex-Fabry M, et al: Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch Intern Med 2006; 166:12801287 Mount Sinai Serials Bibliographic Links [Context Link]
111. Viscusi ER, Reynolds L, Chung F, et al: Patient-controlled transdermal fentanyl hydrochloride vs. intravenous morphine pump for postoperative pain: A randomized controlled trial. JAMA 2004; 291:13331341 Mount Sinai Serials Buy Now Bibliographic Links [Context Link]
112. Chelly JE, Grass J, Houseman TW, et al: The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: A multicenter, placebo-controlled trial. Anesth Analg 2004; 98:427433 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
113. Walder B, Schafer M, Henzi I, et al: Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesth Scand 2001; 45:795804 Mount Sinai Serials Buy Now Bibliographic Links [Context Link]
114. Overdyk FJ, Carter R, Maddox RR, et al: Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg 2007; 105:412418 [Context Link]
115. Cashman JN, Dolin SJ: Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth 2004; 93:212223 Mount Sinai Serials Buy Now Bibliographic Links [Context Link]
116. Ballantyne JC, Carr DB, Chalmers TC, et al: Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth 1993; 5:182193 Mount Sinai Serials [Context Link]
117. Bird M: Acute pain management: A new area of liability for anesthesiologist. ASA News 2007:71. [Context Link]
118. Weinger M: Dangers of postoperative opioids. APSF News 2007; 21:6168 [Context Link]
119. Celerier E, Laulin JP, Corcuff JB, et al: Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: A sensitization process. J Neurosci 2001; 21:40744080 Mount Sinai Serials Bibliographic Links [Context Link]
120. Koppert W, Schmelz M: The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol 2007; 21:6583 [Context Link]
121. Remy C, Marret E, Bonnet F: State of the art of paracetamol in acute pain therapy. Curr Opin Anaesthesiol 2006; 19:562565 Ovid Full Text Mount Sinai Serials [Context Link]
122. Elia N, Lysakowski C, Tramer MR: Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 2005; 103:12961304 [Context Link]
123. Dembo G, Park SB, Kharasch ED: Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology 2005; 102:409415 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
124. Levesque LE, Brophy JM, Zhang B: The risk for myocardial infarction with cyclooxygenase-2 inhibitors: A population study of elderly adults. Ann Intern Med 2005; 142:481489 Mount Sinai Serials Bibliographic Links [Context Link]
125. Grosser T, Fries S, FitzGerald GA: Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest 2006; 116:415 Mount Sinai Serials Bibliographic Links [Context Link]
126. Joshi GP: Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North Am 2005; 23:185202 Mount Sinai Serials [Context Link]
127. Hayashida K-I, Parker R, Eisenach JC: Oral gabapentin activates spinal cholinergic circuits to reduce hypersensitivity after peripheral nerve injury and interacts synergistically with oral donepezil. Anesthesiology 2007; 106:12131219 [Context Link]
128. Hurley RW, Cohen SP, Williams KA, et al: The analgesic effects of perioperative gabapentin on postoperative pain: A meta-analysis. Reg Anesth Pain Med 2006; 31:237247 Mount Sinai Serials Bibliographic Links [Context Link]
129. Bone M, Critchley P, Buggy DJ: Gabapentin in postamputation phantom limb pain: A randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med 2002; 27:481486 Mount Sinai Serials Bibliographic Links [Context Link]
130. Dirks J, Fredensborg BB, Christensen D, et al: A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology 2002; 97:560564 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
131. Himmelseher S, Durieux ME: Ketamine for perioperative pain management. Anesthesiology 2005; 102:211220 Ovid Full Text Mount Sinai Serials Bibliographic Links [Context Link]
Hemodynamics of ketamine for induction in shock patients
Neuroleptic doses of droperidol inhibit sympathomimetic effects of ketamine (Anesth and Analg 1983;62(2):193)
Cardiac Dysfunction
Anaesth Intensive Care. 1997 Jun;25(3):255-9
Failure to successfully complete a procedure following emergency department sedationDMcD Taylor1,2 for the Emergency Department Sedation Study Investigators1Austin Health; 2University of Melbourne, Melbourne, Australia
Aims: To determine the nature and incidence of, and factors contributing to, failure to successfully complete a procedure fol- lowing sedation in the ED
Methods: Eleven Australian ED enrolled consecutive adult and paediatric patients between January 2006 and December 2008. Patients were included if a sedative drug was administered for an ED procedure. Data collection was prospective and employed a specifically designed form.
Results: Two thousand six hundred and twenty three patients were enrolled (60.3% male, mean age 39.2 years). Failure to successfully complete the procedure occurred in 148 (5.6%) cases. Most failures occurred with attempted reductions of fractured/dislocated shoulders (35 cases), hips (32), ankles (21) and elbows (14). However, failure rates were highest among fractured/dislocated hips (18.5%), digits (13.7%), femurs (11.1%), mandibles (10.2%) and elbows (9.3%). Failure rates for residents/registrars (5.9%), consultants (5.6%) and nurse practitioners (5.9%) did not differ (P = 0.92). Overall, failure rates for the various drugs (used alone or in combina- tion) did not differ (P = 0.07). However, ketamine (used alone or in combination) was associated with a much lower failure rate (2.9%) than all other sedation drugs used (midazolam 5.8%, propofol 6.5%, fentanyl 6.9%, nitrous oxide 7.1%, and morphine 7.8%).
Conclusion: Procedural failure is uncommon although some pro- cedures are at higher risk, especially dislocated hip reduction. Failure rates do not appear to be affected by the designation of the operator or the sedative drug used. However, ketamine use is associated with lower failure rates. For those procedures at higher risk of failure, the provision of optimal conditions (spe- cialist unit assistance, venue, drug selection) may minimise failure rates.
Emergency Medicine Australasia 2010;22(S1):A52-3
The cardiorespiratory patterns of critically ill patients brought to the ORand induced with Ketamine. It seems any hypertension is from increased CO and not from increased SVR to any sig. extent. (Crit Care Med 1983;11(9):730)
Ketofol
Ketofol (1:1 mixture of ketamine 10 mg/mL and propofol 10 mg/mL) was administered intravenously at the discretion of the treating physician by using titrated aliquots. [Ann Emerg Med. 2007;49:23-30.]
no real benefits over propofol alone (Ann Emerg Med 2011;57:435)
Etomidate
.1 mg/kg Etomidate up to three doses with fentanyl (Annals EM 40:5, 2002)
others use .15 mg/kg x 2 or go right with .3 mg/kg
Consider combo of fentanyl and droperidol (INNOVAR)
(4) Dursteler BB, et al. Etomidate-facilitated hip reduction in the emergency department Acad Emerg Med 2000;7: 1165-6. (5) Frymann SJ, et al. Reduction of dislocated hip prosthesis in the emergency department using conscious sedation: a prospective study Emergency Medicine Journal 2005;22:807-809
low dose versed (0.015 mg/kg) given 90 sec before etomidate attenuates the myoclonus and doesn’t prolong time to recovery. (Anesth Analg 2007;105:1298)
The question of ketamine “flashbacks” is valid as ketamine is a phencyclidine related to lysergic acid (LSD). Ketamine interferes with the formation of long-term memory at the level of the hippocampus and can thus generate delusionalmemories that are the basis of “flashbacks”. However this requires the generation of a previous short-term memory that is inhibited by co- administration of a GABA agonist, most commonly midazolam. My commonest mix is ketamine 50 mg (5ml of 10mg/ml) and midazolam 5mg (5ml of 1mg/ml). A 2ml initial bolus followed by 1ml every 5-10 min will provide comfort for a wide variety of procedures with no risk of long term effects and very reliable amnesia.
Propofol
Add 30 mg of ephedrine to each 20 cc of propofol for decreased pain of injection and better hemodynamic profile (J Clin Anesth 2009;21:44)
Preloading with fluids doesn’t prevent BP drop (Intensive Care Med (2013) 39:1377–1385)
Initial bolus: Various Guidelines say 0.5 to 2 mg / kg bolus. I would recommend sticking with the lower end 0.5-1 mg/kg To make it even easier just give 50 mg bolus to an average size adult; The recommendations on drip rates are all over the place ranging from 10 mcg/kg/min to 200 mcg/kg/min. I think 10 mcg/kg/ min is too low and a more reasonable starting dose is 100 mcg/kg/min and then titrate upwards by 20 mcg/kg/min every 5 min. I saw one protocol by GI docs for colonoscopy which started at 140 mcg/kg/min. If they can started at 140 mcg/kg/min without complications then I feel confident that our ED docs can start at 100 mcg/kg/min and deliver safe sedation. So to summarize give 0.5-1 mg/kg bolus (50 mg is fine for most adults) followed by a drip at 100 mcg/kg/min. Titrate upward by 20 mcg/kg/min q 5 min.
Soybean oil and egg lecithin are components of the emulsion that contains propofol. Hypersensitivity to these components or the drug itself contraindicates use of the drug as presently formulated. Allergy to eggs alone, is probably safe (
Emerg Med J
2013;30:79-80)
(Academic EM 10:9 931-937, Sept 2003) Randomized Clinical Trial of Propofol versus Methohexital for Procedural Sedation during Fracture and Dislocation Reduction in the Emergency Department: 1 mg/kg then 0.5 mg/kg Q 3-5 minutes
Propofol (2,6-diisopropylphenol), an oil in water emulsion, is an intravenous agent that is used for induction and maintenance of anesthesia.7 Propofol, like benzodiazepines, inhibits activity at both the spinal and supraspinal synapses by interacting with and potentiating the GABA-mediated receptors.8,9 However, it does not potentiate GABA-evoked currents through the benzodiazepine site.10 When combined with flurazepam, the potentiation of GABA receptor activity obtained with propofol is greater than expected from a simple additive response.
Intravenous injection of propofol produces a rapid hypnosis, usually by 40 seconds after the start of a bolus injection. Maintenance of sedation (25 to 75 µg/kg/minute) or anesthesia (100 to 200 µg/kg/minute) can be achieved by continuous intravenous infusion titrated to clinical effect.7 Propofol distributes very rapidly throughout the body including the brain. The pharmacokinetic profile of propofol is described by a three-compartment model:
Propofol Infusion Syndrome
Sedation for Cardioversion Study
(Annals EM Dec 2003 42:6)
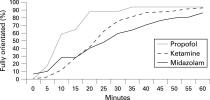
Propofol (9) vs. Etomidate (9) vs. Midazolam (8) vs. Midazolam with flumazenil (6)
Recovery Etomidate 9.5 minutes, Midazolam 21 minutes, Propofol 8 minutes, Midaz c flumaz 3 minutes but high resedation rate
Flumaz protocol was .5 mg bolus then .5 mg over one hour
Etomidate .2 mg/kg
Propofol 1.5 mg/kg
Midazolam .2 mg/kg
Hemodynamic effects of propofol sedation (Anesthesiology 2005;103:20)
It has substantial inhibitory effects on sympathetic activity and reflex responses to hypotension
this causes vasodilation and decreased MAP
Study using infusion instead of injection (Am J Emerg Med. 2006 Sep;24(5):599-602)
Because of propofol’s lack of analgesic activity, fentanyl was given intravenously at a dose of 2 μg/kg approximately 2 minutes before beginning the propofol infusion. Propofol was then begun as an infusion using a pump. Initially, a loading infusion of 0.21 mg kg−1 min−1 was given until the patient was sedated to an appropriate level, assessed by the attending emergency physician. At this point, the procedure was started, and the propofol infusion was switched to a maintenance rate of 3-6 mg kg−1 h−1, at the attending physician’s discretion, until the procedure was completed. A table with appropriate doses and drip rates was compiled and available at all times during the procedure Etomidate and propofol appear equally safe for ED procedural sedation; however, etomidate had a lower rate of procedural success and induced myoclonus in 20% of patients. (Annals of Emergency Medicine Volume 49, Issue 1 , January 2007, Pages 15-22) Anaesthesia Volume 62 Issue 7 Page 690Issue 7 – 701 – July 2007 To cite this article: P. C. A. Kam, D. Cardone (2007) Propofol infusion syndrome Propofol infusion syndrome (Anaesthesia 62 (7), 690701.)
We read with interest the recent correspondence highlighting the inclusion of peanut allergy in the list of contra-indications in the product literature for propofol [1]. The potential for peanut-allergic patients to be sensitive to propofol is due to the fact that soya oil is one of the excipients of propofol, and that cross-reactivity occurs in legume allergy, for example between soya and peanut. However, it is the protein component that is responsible for allergic reactions in these patients, and as the soya oil present in propofol is refined, it is unlikely to contain significant quantities of allergenic particles. In the food industry soya-containing mixed vegetable oil may be sold for consumption without listing soya as an ingredient [2]. The reason for this is that the dose of protein contained in refined soya oil is too small to provoke a reaction when ingested. Although the minimum dose of protein required to trigger a reaction after oral ingestion has been identified, the same is not true of parenteral administration [3].
There is no mention of egg allergy in the product literature for propofol. The main triggers for egg anaphylaxis are three proteins found in egg white: ovoalbumin, ovomucoid and conalbumin. Lecithin, a purified egg phosphatide present in propofol, is not thought to be problematic for patients who are allergic to eggs. Allergic reactions to propofol have been shown to be triggered by the iso-propyl or phenol groups rather than the lipid vehicle [4, 5].
One case report describes an anaphylactic reaction following administration of propofol to a child with multiple food allergies including egg and peanut. However, other drugs including rocuronium had also been administered. Skin prick testing was not performed and the specific causative agent remains unclear [6]. We have spoken to the Medical Information Department at AstraZeneca who informed us that the final decision to use Diprivan in patients with egg allergy remains at the discretion of the individual anaesthetist concerned (personal communication, D. Gupta, AstraZeneca Ltd).
In summary, the negligible protein content of refined soya oil in propofol suggests the drug is unlikely to trigger a reaction in patients with peanut allergy. The fact that the product literature cites this as a contra-indication must, however, be given careful consideration prior to administration. If the clinician decides propofol is unsuitable, inhalational induction of anaesthesia may provide a useful alternative.
Xenon
May have potential as an inhalational sedative without hemodynamic side effects (Crit Care Med 2003 31:10)
We can sedate the critically ill, unstable patient (Acad Emerg Med 2005;12(2):124)
0.5 mg/kg of propofol plus 0.5µg/kg of remifentanil, given intravenously over 60 and30 seconds, respectively for shoulder dislocation (EMJ 2006;23:57-58)
capnography for procedural sedation (Ann Emerg Med 2007;50:172) review of propofol sedation (Ann Emerg Med 2007;50:182)
Flumazenil
clinical effects 30-60 min duration
0.5-5 mg infused over 3-5 min
Propofol
avoid using in egg, soybean, or EDTA allergy
interacts with GABA receptor system
prolongs duration of contact between gaba and its receptor site
liver metabolized
duration ~8 minutes
antiemetic qualities
rapid IV bolus causes higher incidence of resp depression
ketamine/propofol combination may mitigate the cardiovascular effects
cat b in pregnancy
0.5 mg/kg of lidocaine mixed up with propofol will limit pain on injection
for sedation consider giving 10% of induction (1.5 mg/kg) dose
ketamine + propofol
5 mg/cc solution of each titrate 1-2 cc at a time
can be placed in the same syringe
Etomidate
premed with opioids or benzos may decrease myoclonus
or you can use magnesium The following article reported decreased myoclonus with Mg++ pretreatment. I believe the authors used 100 mg Mg++. I have found that 300-500 mg seems to work better with younger patients and higher doses of etomidate. There may still be a small amount of twitching, but greatly reduced.Anesth Analg 2005;101:705-709
use with lidocaine to decrease injection burning
ADD lidocaine to sedation checklist
inhibition of gaba neurotransmission
redistribution from brain to peripheral tissues accounts for its short action, though eventual metabolism is by liver
1/3 of patients will have myoclonus
dantrolene 1mg/kg can terminate severe myoclonus
propylene glycol is diluent
Ketamine
no pain on injection
now known pretreatment with benzos has no benefit
include fall precautions on d/c instructions
inhibits gaba, halmoneocortical projection system, NDMA and mu agonist
arylcyclohexylamine resembling PCP
liver metabolized
increases cardiac output
benzos delay ketamine metabolism prolonging action
cat B in pregnancy (though sources have various listings)
1.5 mg/kg, IM dosing is 4 mg/kg
Benzodiazepenes
nitroglycerin like effect on heart fx paitents which reduces ventricular filling
hepatic p450 metabolism
increase frequency of cl channel opening
ativan and versed essentially have the same dosing with 2 of either = to about 5 of valium
ativan has no active metabolites, diazepam and versed do. Kidney fx will cause prolonged action
paradoxical agitation
class D in pregnancy
Frequent hypoxemia and apnea after sedation with midazolam and fentanyl (Anesthesiology 1990;73:826) Midazolam alone had no resp depressant effects. Fentanyl alone produced hypoxemia but not apnea. Midaz + fentanyl had sig. increased rate of hypoxemia.
ETCO2
ETCO2 monitoring makes procedural sedation safer (Acad Emerg Med Volume 13, Number 5 500-504)
Meta-Analysis of capnography during procedural sedation (J Clin Anesth 2011;23:189)
17.6 x more likely to detect resp depression if ETCO2 is used
Supplemental Oxygen
They summarise:
“…assuming that capnography is in place to monitor ventilatory function, our results strongly support the routine use of high-flow oxygen during ED propofol sedation”
STUDY OBJECTIVE: We determine whether high-flow oxygen reduces the incidence of hypoxia by 20% in adults receiving propofol for emergency department (ED) sedation compared with room air.
METHODS: We randomized adults to receive 100% oxygen or compressed air at 15 L/minute by nonrebreather mask for 5 minutes before and during propofol procedural sedation. We administered 1.0 mg/kg of propofol, followed by 0.5 mg/kg boluses until the patient was adequately sedated. Physicians and patients were blinded to the gas used. Hypoxia was defined a priori as an oxygen saturation less than 93%; respiratory depression was defined as an end tidal CO(2) greater than 50 mm Hg, a 10% absolute change from baseline, or loss of waveform.
RESULTS: We noted significantly less hypoxia in the 59 patients receiving high-flow oxygen compared with the 58 receiving compressed air (19% versus 41%; P=.007; difference 23%; 95% confidence interval 6% to 38%). Respiratory depression was similar between groups (51% versus 48%; difference 2%; 95% confidence interval -15% to 22%). We observed 2 adverse events in the high-flow group (1 hypotension, 1 bradycardia) and 2 in the compressed air group (1 assisted ventilation, 1 hypotension).
CONCLUSION: High-flow oxygen reduces the frequency of hypoxia during ED propofol sedation in adults.
The Utility of High-Flow Oxygen During Emergency Department Procedural Sedation and Analgesia With Propofol: A Randomized, Controlled Trial
Ann Emerg Med. 2011 Oct;58(4):360-364
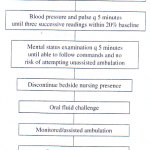
When to Discharge
Remifentanil
remifentanil review article
Hyperoxia exacerbates opioid-induced resp depression (Br. J. Anaesth. (2013) 110 (5): 837-841.)
NIPPV
from Strayer et al (Am J Emerg Med 2015;33:108)